Biosensors
A major research focus of this lab is the design and development of novel sensing tools for use in healthcare and environmental settings.
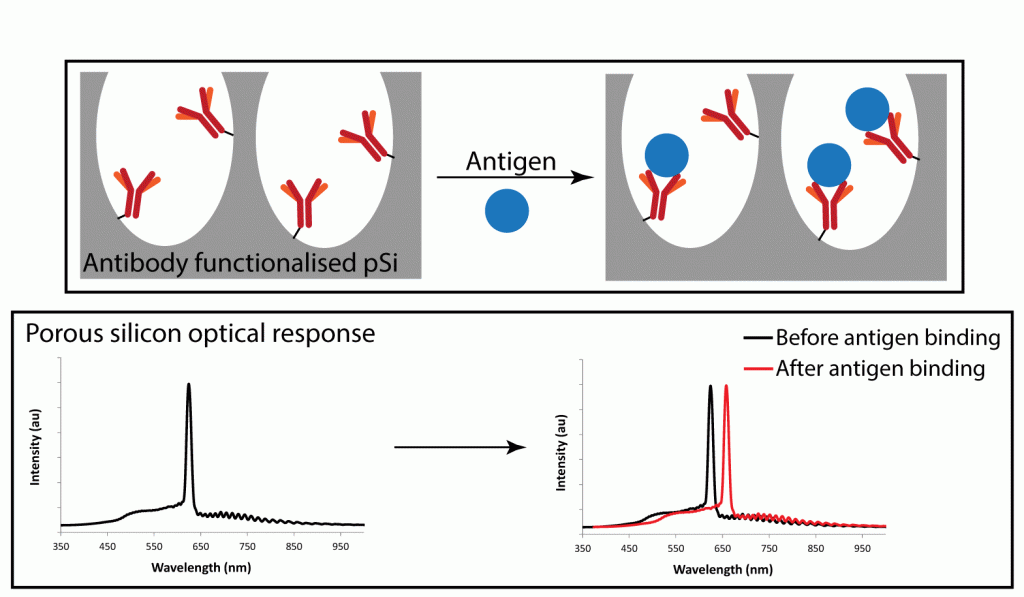
 Porous silicon composites can be fabricated to report the presence of an analyte or environmental parameter by a change in colour. This phenomenon has been harnessed in the development of several prototype healthcare materials for use in wound management. Smart bandages that change colour when changes in pH and temperature occur in a wound can monitor inflammation or infection. Novel porous silicon biosensor systems have been designed to capture a range of clinically relevant molecules from small volumes of wound fluid. Our lab also explores porous silicon biosensors in veterinary applications.
Porous silicon composites can be fabricated to report the presence of an analyte or environmental parameter by a change in colour. This phenomenon has been harnessed in the development of several prototype healthcare materials for use in wound management. Smart bandages that change colour when changes in pH and temperature occur in a wound can monitor inflammation or infection. Novel porous silicon biosensor systems have been designed to capture a range of clinically relevant molecules from small volumes of wound fluid. Our lab also explores porous silicon biosensors in veterinary applications.
In complementary work, we also develop electrochemical biosensors that use nanostructured materials as transducers. In a collaborative project with SA Water devoted to the rapid, sensitive and reliable detection of key microorganisms and disinfection by-products in water, we combine the outstanding performance of porous transducer materials, such as porous silicon, with non-conventional bacteriophage receptors. This is expected to lead to low-cost platforms suitable for a wide range of contaminants. We also work in close collaboration with cardiologists to develop point-of-use devices to rapidly diagnose cardiac infarct. Polymer membranes coated with ultrathin semiconducting or conducting films act as transducers, able to detect and amplify molecular signals that occur during a cardiac event. Patients at risk of recurrent cardiac events can be closely monitored with the tool, allowing treatment strategies to modified for maximum effect.
Porous silicon in drug delivery
Nanomaterials promise to improve disease diagnosis and therapy through effective delivery of drugs, genes, biomolecules and imaging agents. The use of silicon based nanomaterials for targeted drug delivery forms a major research focus of this laboratory.
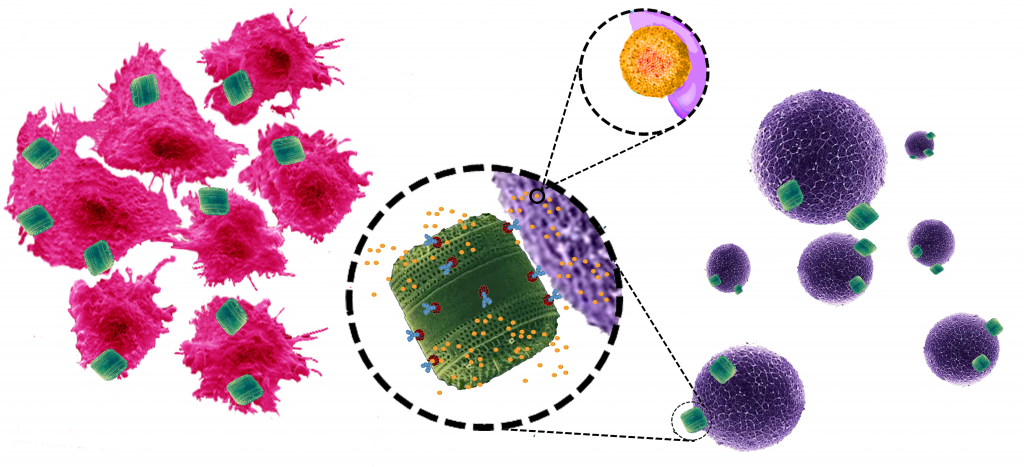
Porous silicon is a high surface area, biocompatible and bioresorbable form of silicon, widely employed in biomedical applications including drug delivery of proteins, enzymes, small molecular drugs and nucleic acids. By functionalisation of the porous silicon surface, the kinetics of drug release can be controlled. We investigate coatings responsive to temperature and pH as a strategy to deliver defined drug doses over an extended length of time directly to their site of action. As an example, we have designed smart dressings that automatically release a drug in response to temperature changes in the wound environment.
Analogous to the way needles are used in macroscopic medicine to transport drugs or diagnostic agents into living tissue, vertically aligned silicon nanowire arrays can be used in micro-scale biological systems to transport biomolecular cargo inside mammalian cells. VAS-NW arrays can be generated with fine control over the dimensions, density, location and orientation of the nanowires. We engineer the surface chemistry of these nanowire arrays to control the rate of degradation while maximizing the efficiency of the gene delivery.
Porous silicon in forensic applications
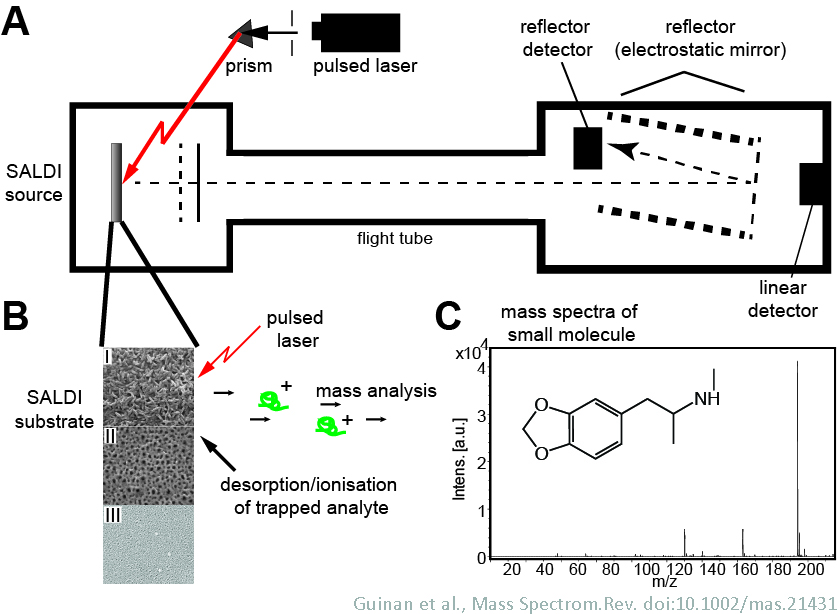
The high surface area and UV absorbing capability of porous silicon has led to its application as a substrate to capture material for MALDI-mass spectroscopic analysis. We have developed lab-on-a-chip onsite drug testing platforms based on porous silicon substrates. In this technique, drug molecules are extracted into porous silicon directly from saliva or fingerprints. These molecules are then desorbed by laser and detected based on their properties within a mass spectrometer. Multiple drugs can be detected within a single sample. Several classes of drug and their metabolites have been successfully detected, ranging from cocaine, amphetamines, methamphetamines and ecstasy to benzodiazepines and opiates. These devices are currently in field trials for use in road side testing. With the advent of small scale mass spectrometry equipment, such devices enable definitive on-site drug testing.
Green technologies
Porous silicon membranes for desalination. Nanostructured materials are extremely well suited for high efficiency treatment of source waters. Particularly exciting are
membrane materials that mimic the selective transport and fast flow rates in biological water channels. These types of materials promise new and energy-efficient means of water filtration. In the current project, we are fabricating ultrathin nanostructured silicon membranes for desalination. These membranes feature monodisperse pore sizes and short transport paths, increasing selectivity and flux. Plasma-based surface engineering can also be incorporated to reduce biofouling, prolonging membrane life and enabling effective removal of toxins.
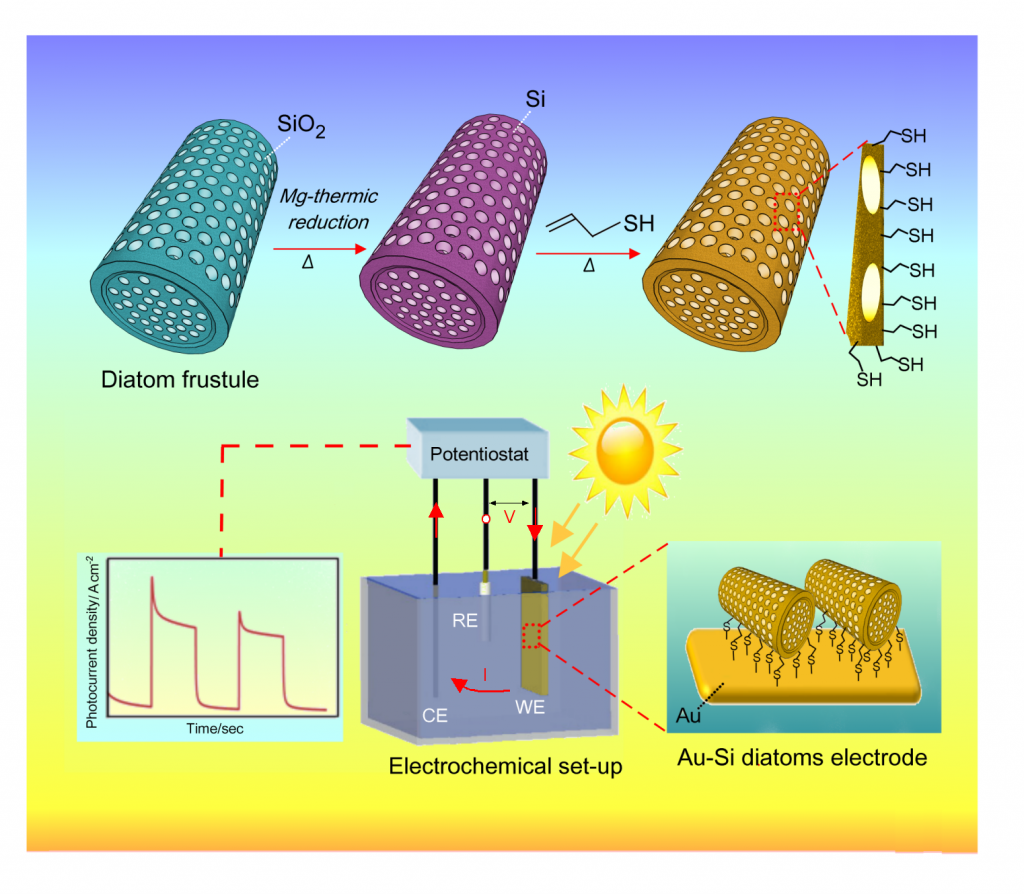
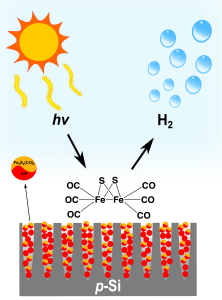 Photoelectrochemical devices from natural systems. Diatoms are naturally occurring nanostructures made from silica (SiO2). We investigate the conversion of diatoms fromsilica (insulating) into silicon (conductive) in order to employ them in photoelectrochemical (PEC) devices. PEC devices work on the principle of artificial photosynthesis, where sunlight and water are used to produce the storable fuel hydrogen. At present, stable currents can be observed when light is shone on modified diatoms attached to a gold electrode. Sensitisation of the diatoms with quantum dots is under investigation to improve the efficiency of this device.
Photoelectrochemical devices from natural systems. Diatoms are naturally occurring nanostructures made from silica (SiO2). We investigate the conversion of diatoms fromsilica (insulating) into silicon (conductive) in order to employ them in photoelectrochemical (PEC) devices. PEC devices work on the principle of artificial photosynthesis, where sunlight and water are used to produce the storable fuel hydrogen. At present, stable currents can be observed when light is shone on modified diatoms attached to a gold electrode. Sensitisation of the diatoms with quantum dots is under investigation to improve the efficiency of this device.
Characterisation of novel plant proteins. Transmembrane ion transporter proteins play a vital function in plant growth, development and survival. Characterisation of a novel borate transporter protein by atomic force microscopy within a model lipid bilayer system has revealed its native conformation. In collaboration with plant biologists at the UniSA Waite campus, we are currently performing ion transport analysis by patch clamp measurements to further elucidate its function. This protein plays a crucial role in the regulation of borate in crops. Understanding the function of the protein may lead to the development of resistant crops that can grow in poor soils.
Surfaces for high throughput screening of cells and tissues
In the Voelcker laboratory, we use two principal surface screening techniques to investigate how cells respond to their environment: cell microarrays and gradient surfaces. These methods allow us to rapidly identify parameters that influence cell survival, differentiation and function. Miniaturisation of the screening platforms maximise the amount of data that can be obtained from limited numbers of cells. Large combinatorial spaces can be screened on a single surface. We have applied these surfaces to look at problems as diverse as the critical density of growth factor molecules needed to support mesenchymal and neural cell differentiation, damage to cells through irradiation, the genotoxicity of ubiquitous nanomaterials and how cell shape can influence cell fate.
Smart materials to support cell therapies
In collaboration with biologists, engineers and physicians, we seek to create new treatments for diabetes, ischemia and reperfusion injuries, and autoimmune disorders based on cell therapies. Building on the identification of key variables in the cell microenvironment recognised using our high-throughput screening methodologies, we design and construct smart materials that support the transport, scaleable expansion and successful transplantation of therapeutic cells. Our expertise in generating biocompatible pSi-polymer composites allows controlled factor and drug release to be incorporated into culture and transplant scaffolds.